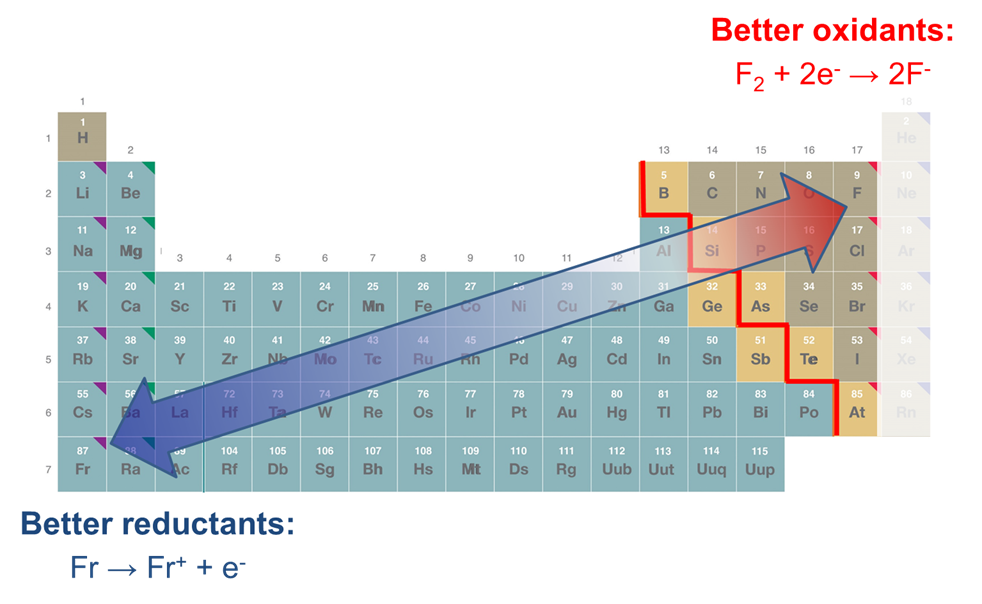
8.1.4: As may be seen from considering an element's redox diagrams, main group elements (aside from the noble gases) generally are more oxidizing towards the upper left of the periodic table and
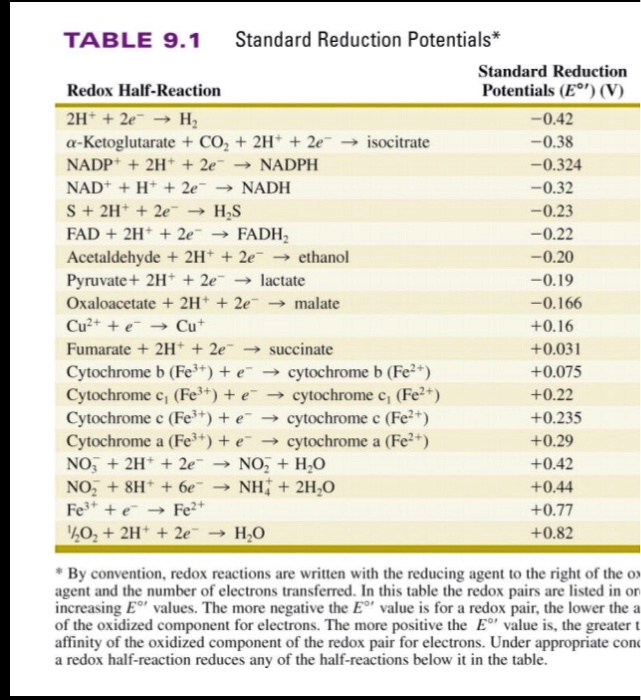
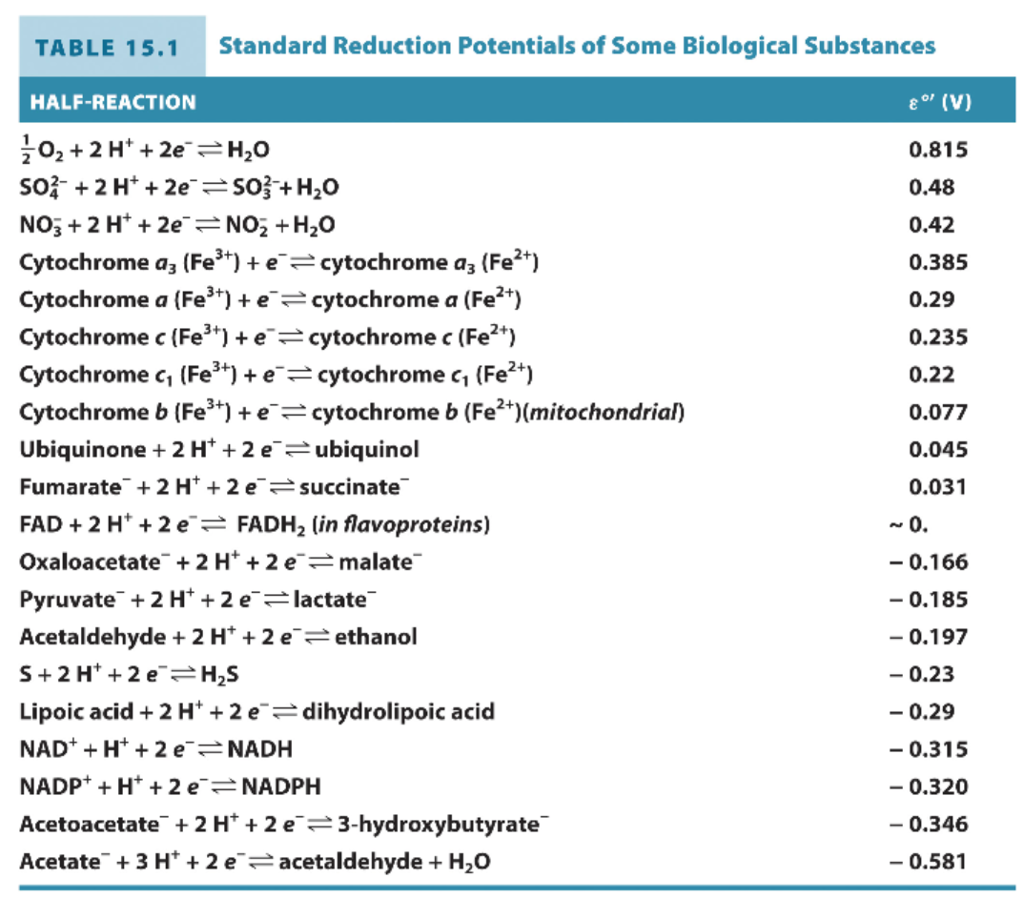
SOLVED: TABLE 9.1 Standard Reduction Potentials* Standard Reduction Redox Half-Reaction Potentials (E" 2H" 2e H; 0.42 Ketoglutarate CO + 2H 2e isocitrate -0.38 NADP 2Ht 20 NADPH 0.324 NAD" HF 20 NADH -
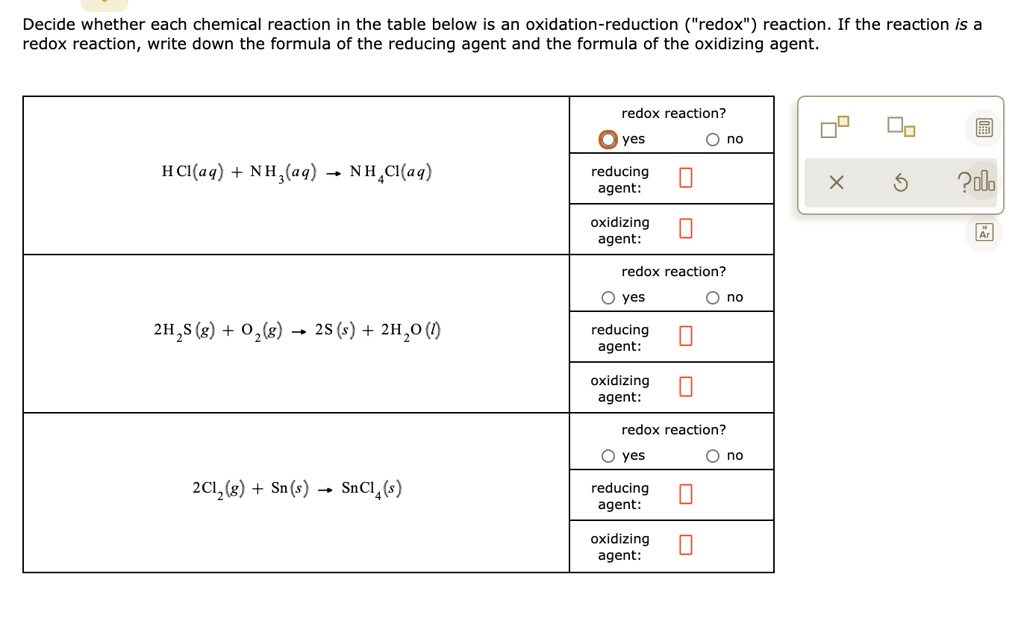
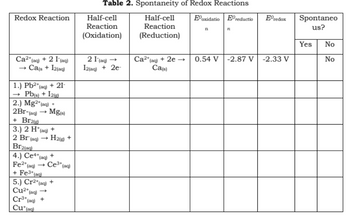
SOLVED: Decide whether each chemical reaction in the table below is an oxidation-reduction "redox' reaction. If the reaction is a redox reaction, write down the formula of the reducing agent and the

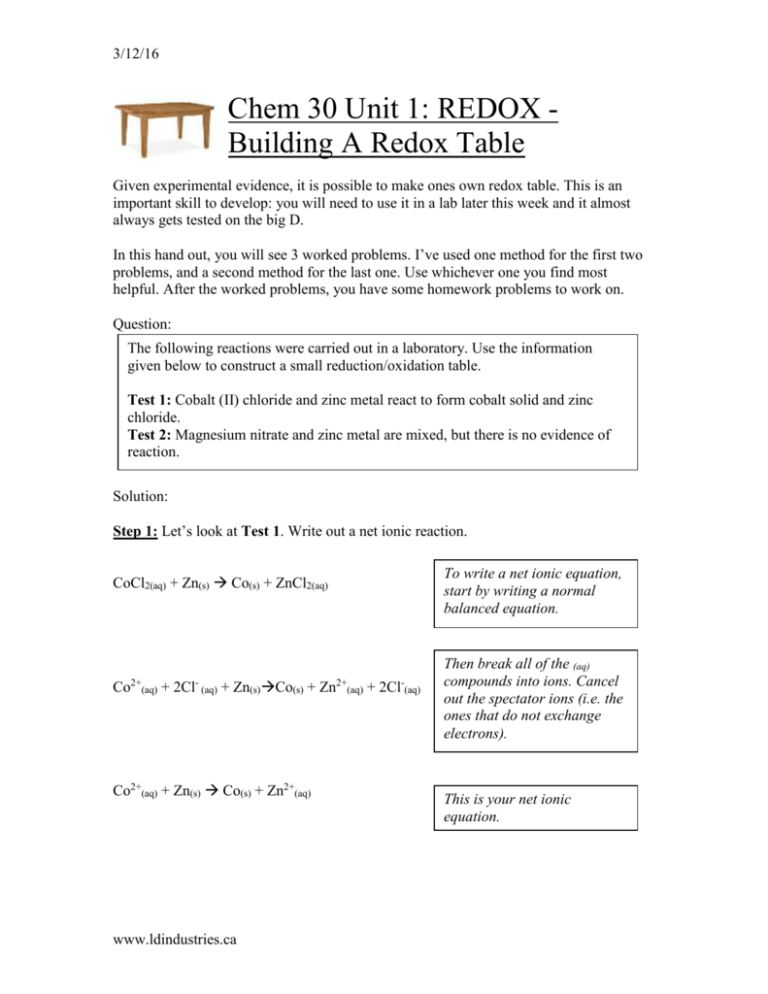
OneClass: Use the Standard Reduction Table to write net ionic equations for the extensive redox react...


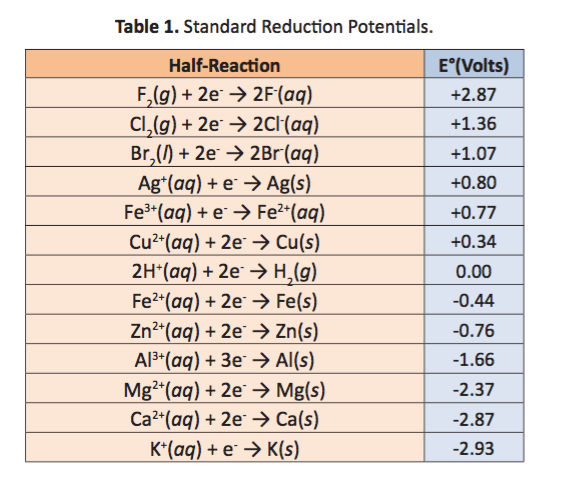



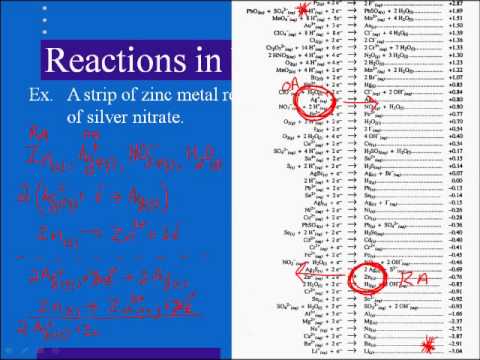
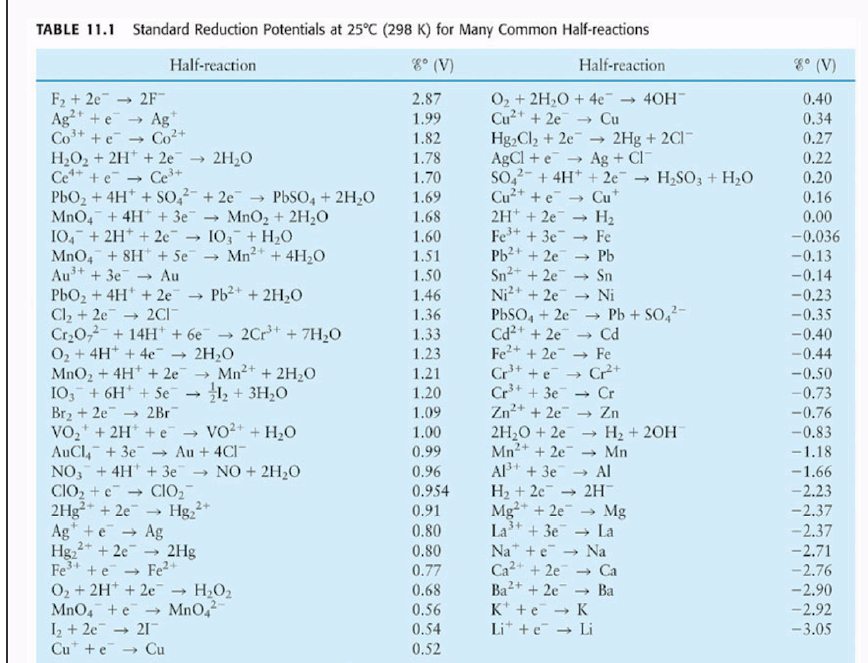

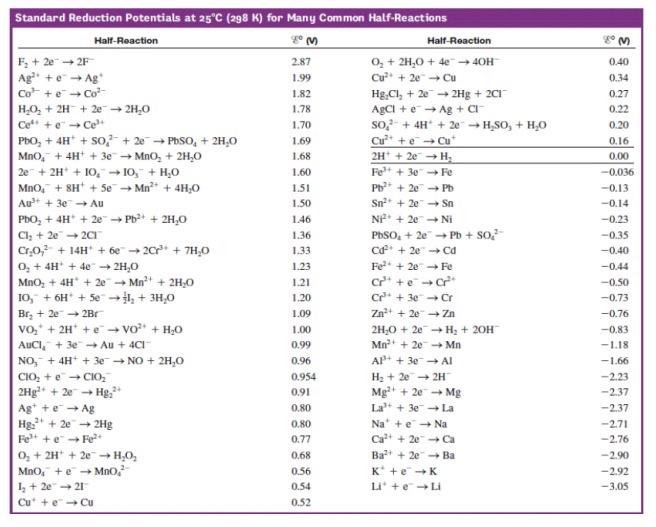
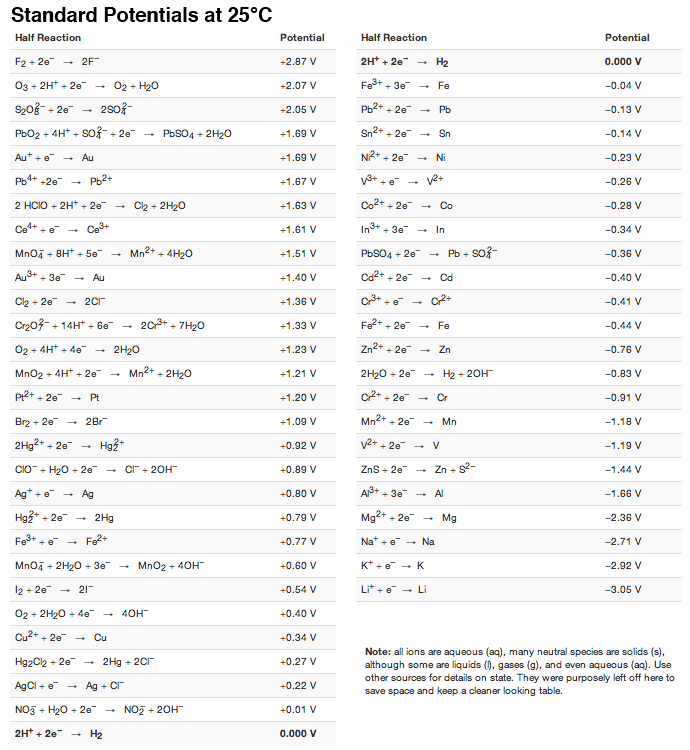






![AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS] AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS]](https://www.wissensdrang.com/media/tablerp.gif)