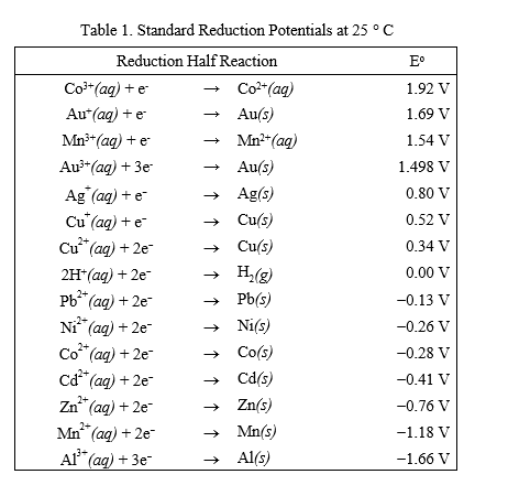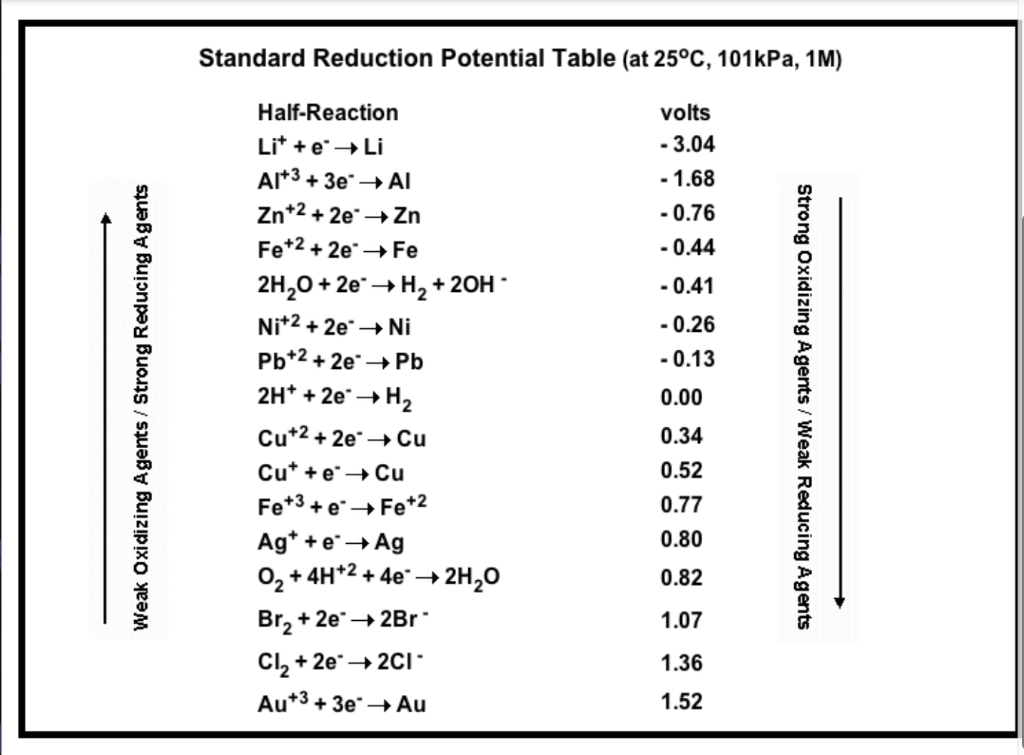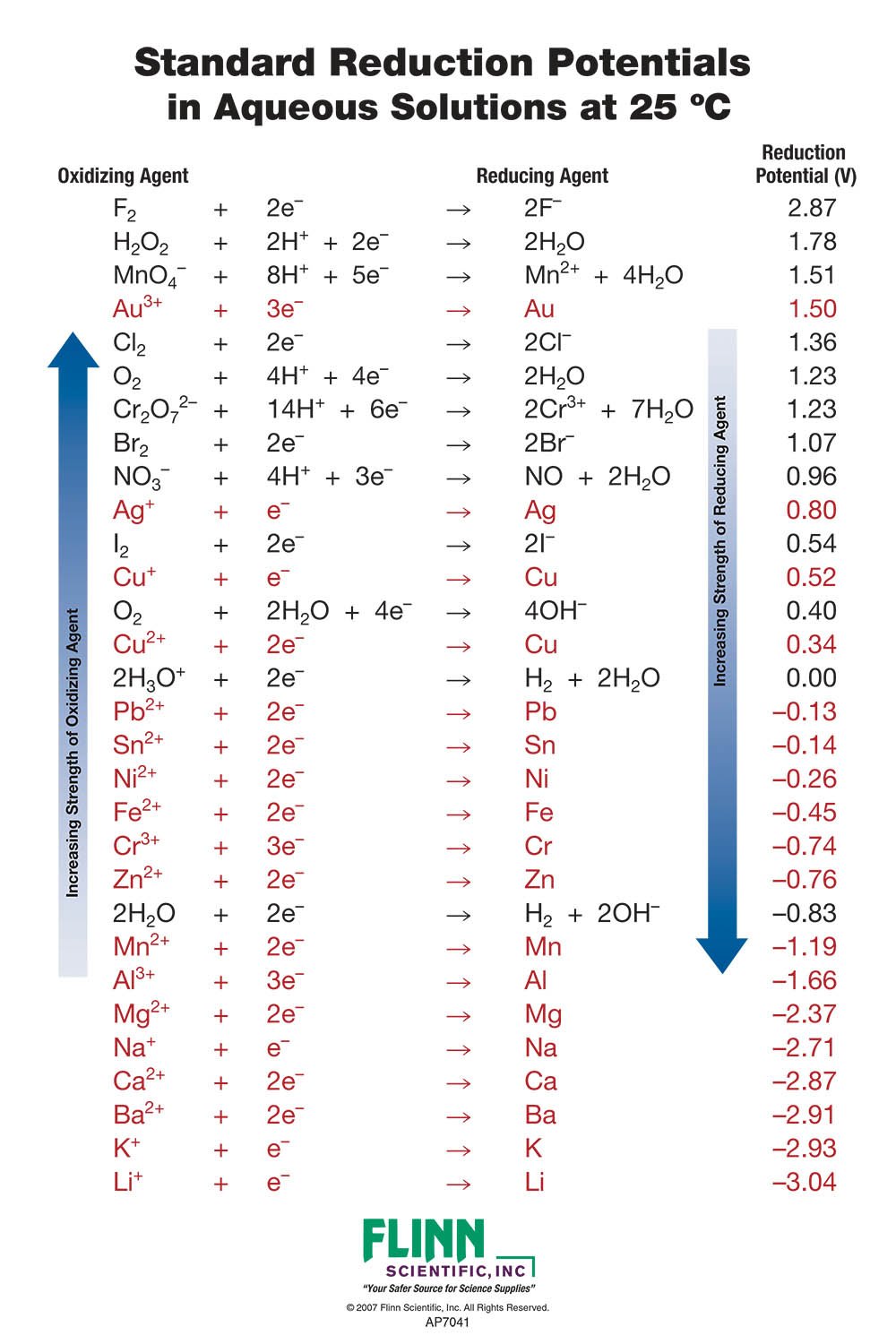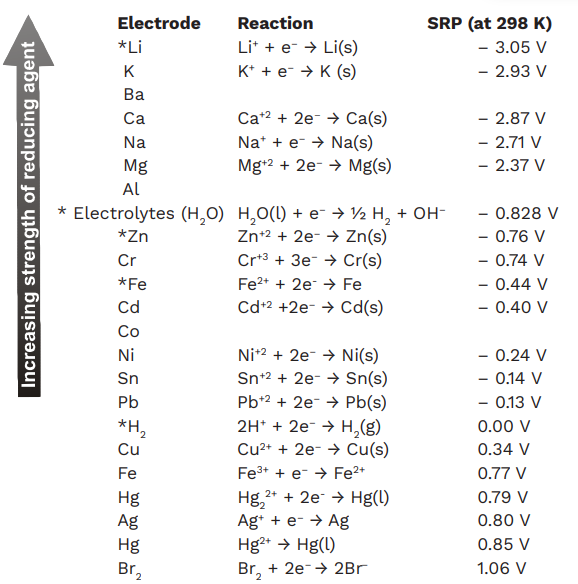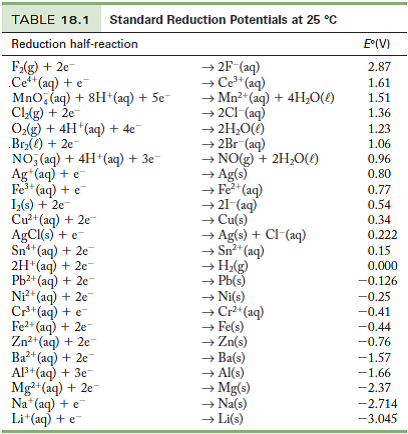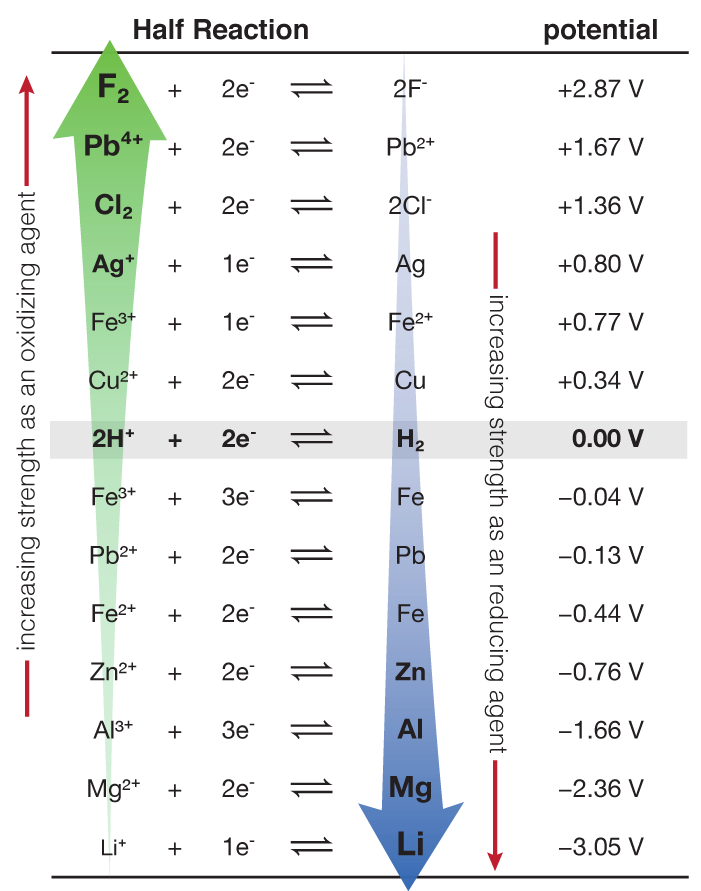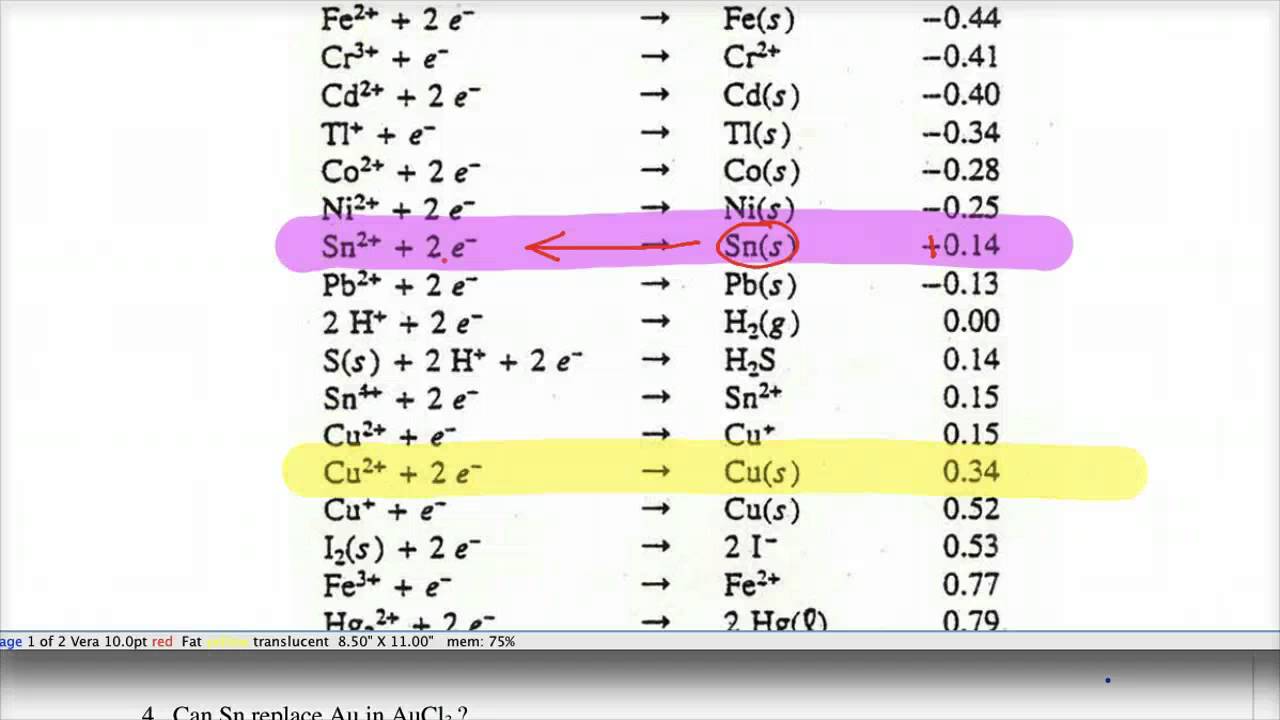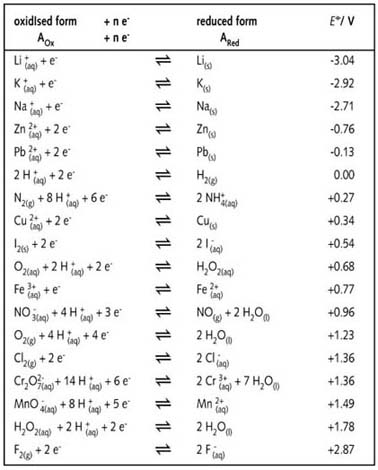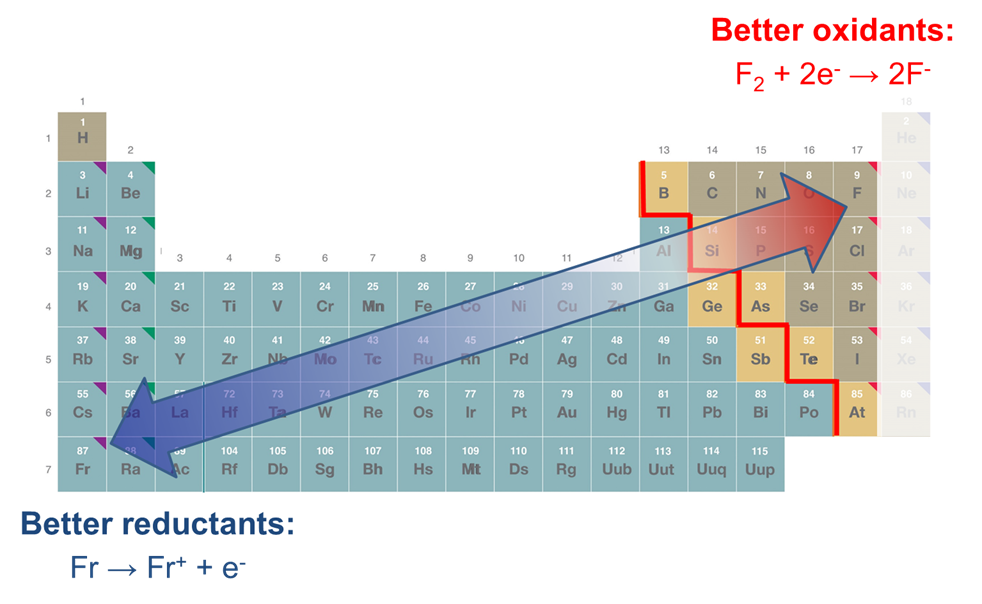
8.1.4: As may be seen from considering an element's redox diagrams, main group elements (aside from the noble gases) generally are more oxidizing towards the upper left of the periodic table and

Table of Standard reduction potentials.pdf - Table of Standard reduction potentials Half reaction Li e Li s K e K s Ca2 2e Ca s Na e | Course Hero

WebElements Periodic Table » Periodicity » Reduction potential of hydrated M(I) ions » Periodic table gallery

SOLVED: Table A5.5 Standard Reduction Potentials at 258C (298 K) for Many Common Half-Reactions Half-Reaction Half-Reaction 4OH Fz + 2e 2F 2.87 01 2H,0 + 4e Cu? + Ze Cu Ag + +

Table 1 from Calculation of Standard Reduction Potentials of Amino Acid Radicals and the Effects of Water and Incorporation into Peptides. | Semantic Scholar
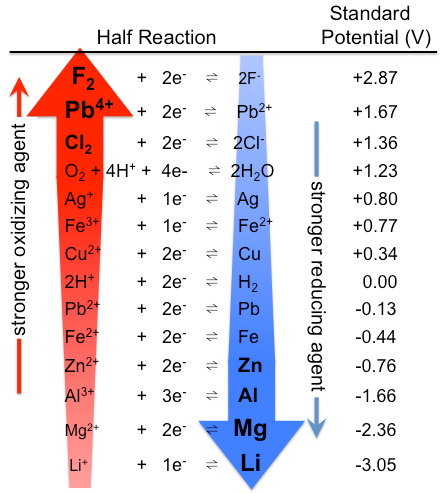
8.1.4: As may be seen from considering an element's redox diagrams, main group elements (aside from the noble gases) generally are more oxidizing towards the upper left of the periodic table and


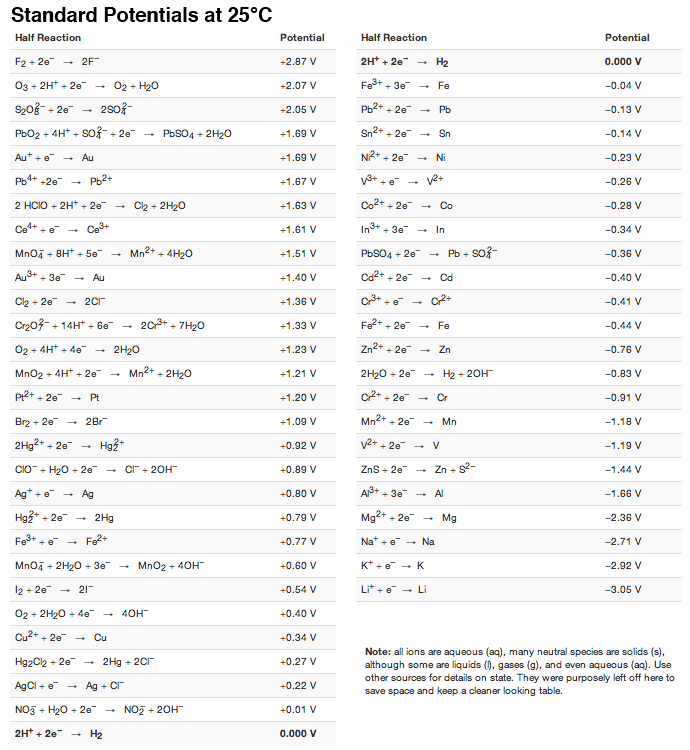

![AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS] AUFBAU1 [REFERENCE SECTION: REDOX POTENTIALS]](https://www.wissensdrang.com/media/tableop.gif)