
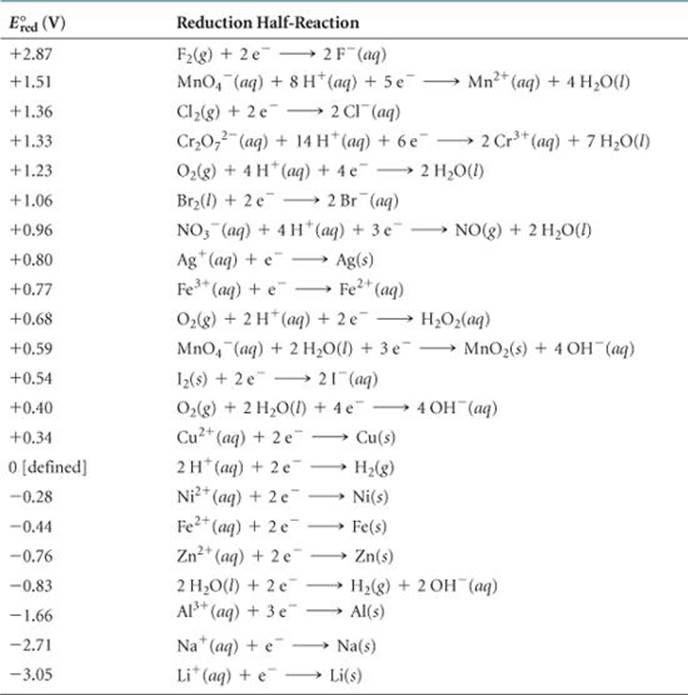
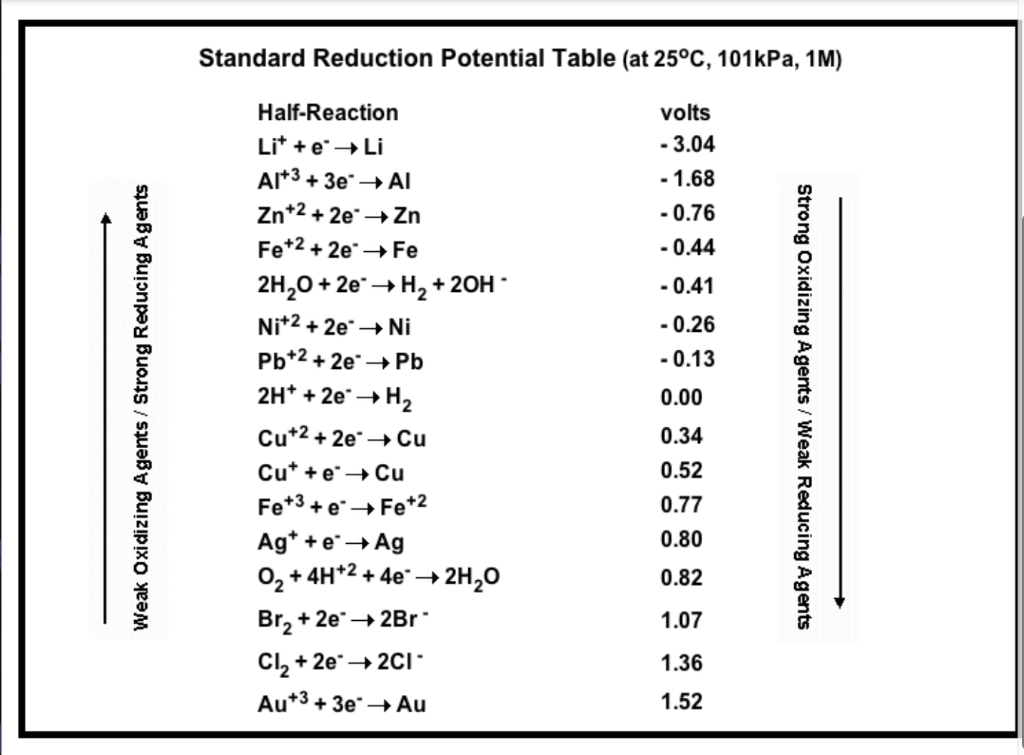
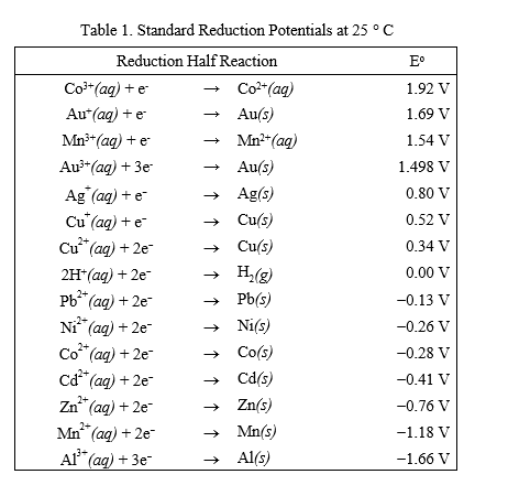
physical chemistry - Explain why can't zinc be plated out from a Zn(II) solution using standard reduction potentials - Chemistry Stack Exchange

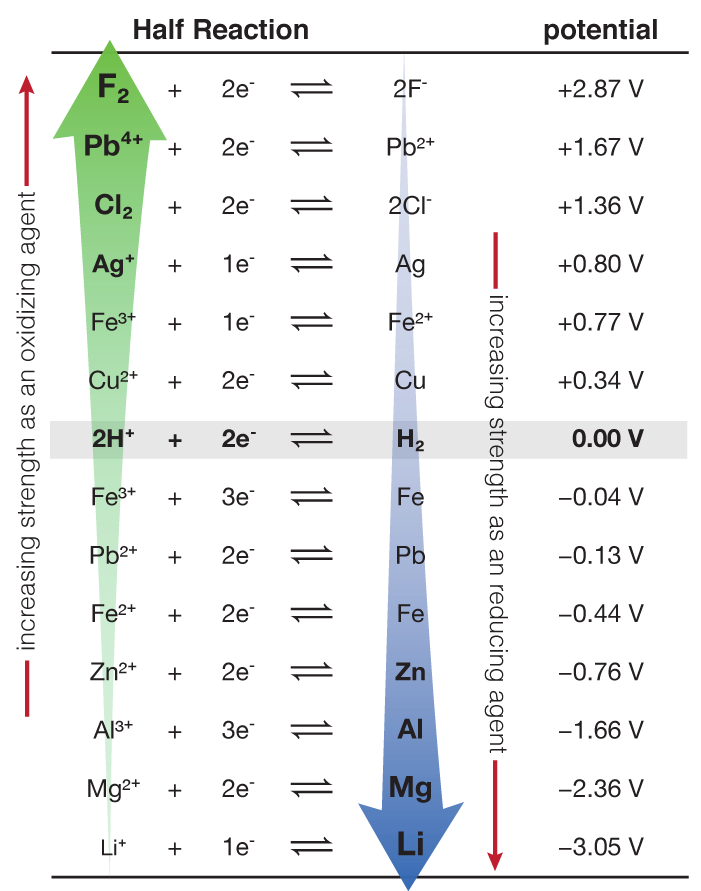
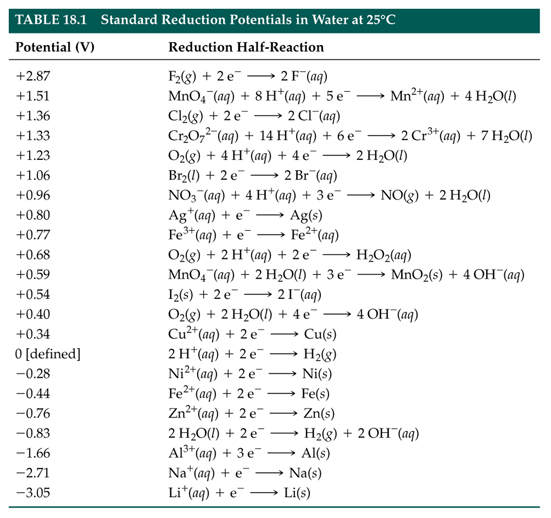
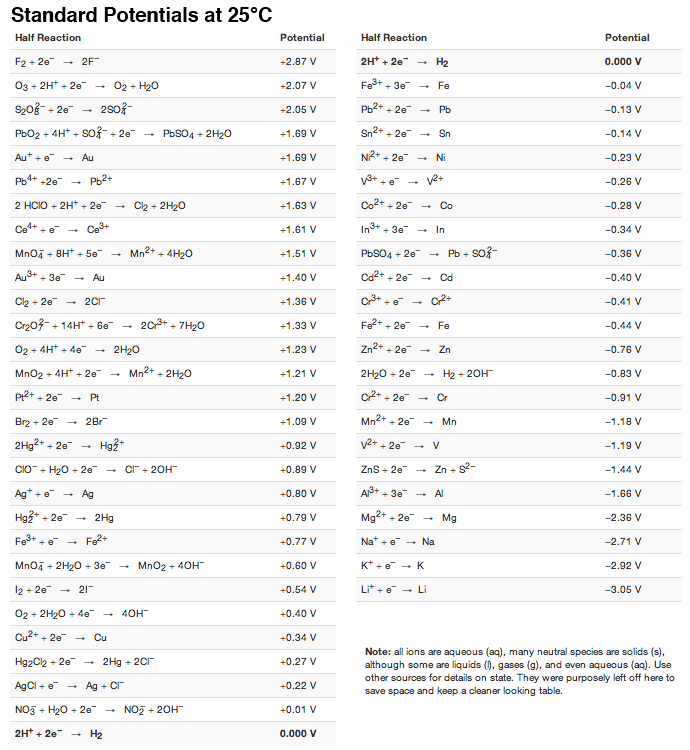
SOLVED: TABLE 17.1 Standard Reduction Potentials at 25 " Reduction Half-Reaction F(g) 2e- HO-(aq) 2 H'(a4) 2e" MnO;-(aq) 8 H" (q) 5e" CI) 2e" Cr,0 "-(aq) 14 H-(aq) 6e" 0,@) 4H* (4q)

Using the standard electrode potentials given in the Table, predict if the reaction between the following is feasible. Ag(s) and Fe^3 + (aq)

Table 1 from Absolute standard hydrogen electrode potential measured by reduction of aqueous nanodrops in the gas phase. | Semantic Scholar



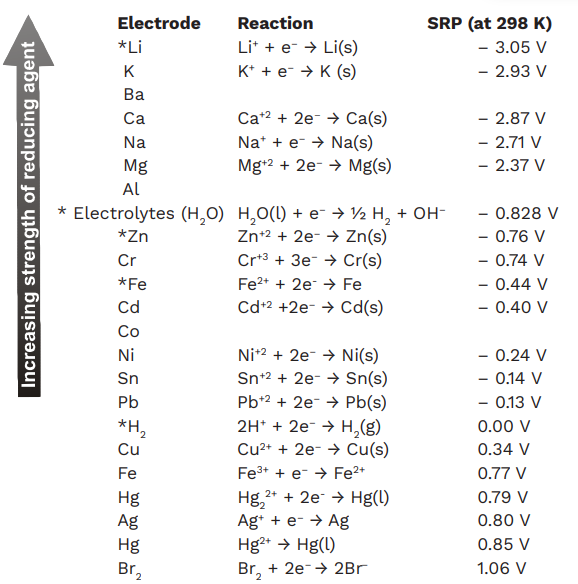
![Standard reduction potentials at 298°K. [24] | Download Table Standard reduction potentials at 298°K. [24] | Download Table](https://www.researchgate.net/publication/316026333/figure/tbl2/AS:650784626708491@1532170554986/Standard-reduction-potentials-at-298K-24.png)


:max_bytes(150000):strip_icc()/Standardreductionpotential-5b551731c9e77c003ec223b3.jpg)




